Multi Material Electrocatalysis
Project heads : Manuel Landstorfer, Jürgen Fuhrmann Project staff : Rüdiger Müller
Goals
Electrocatalysis is a central ingredient for a variety of modern technologies to store, convert and generate electric power.
Important applications are fuel cells and electrolysers, redox flow- and metal-air batteries as well as material synthesis and conversion.
In order to apply these technologies on a scale sufficient for the Energiewende, new catalysts need to be developed which are cheap, non-toxic, durable, processable, and efficient for a specific process.
This requires fundamental insights and new ideas in electrochemistry and electrochemical engineering.
Electrochemistry research depends on a variety of experimental methods to characterize electro-catalytic reactions occurring on the interface between an electrolyte and the conductive material of the catalyst.
The project goal are continuum models for electrocatalysis at the \(nm\) – \(\mu{}m\) scale coupling reactions on catalytic interfaces, reactant transport in electrolytes and charge transport in catalyst substrates. Implementation into numerical simulation tools will support the interpretation of electrochemical measurements.
Results for thermodynamic equilibrium
Double layer capacitance of polycrystalline electrode
First results on modeling, simulation and validation of electrochemical interfaces on polycrystalline materials cover the case of ideally polarizable electrodes. A methodology to predict important characteristics like double layer capacity of an electrode described as an ensemble of grain surfaces from the corresponding information of the individual grain types has been developed.
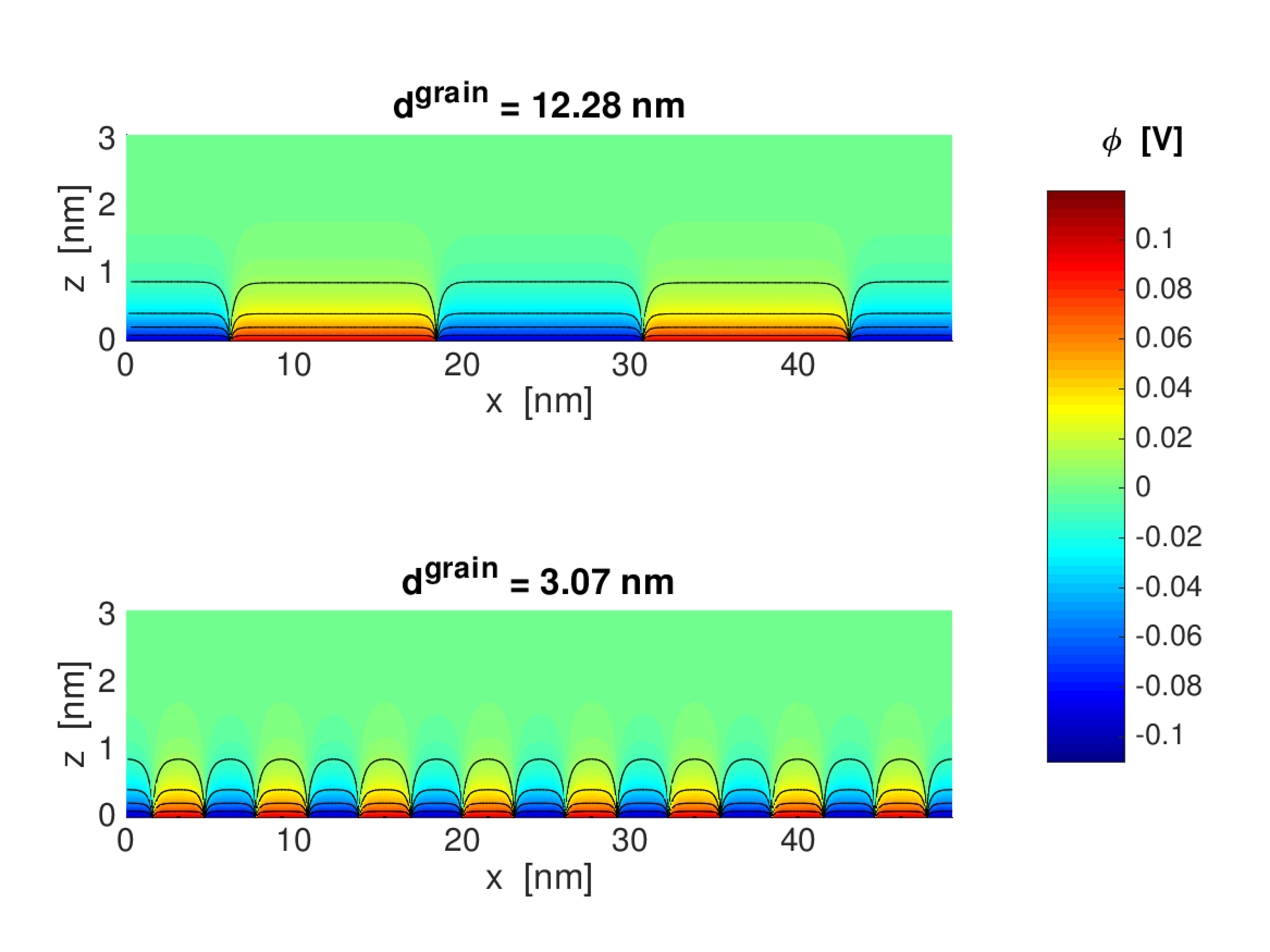
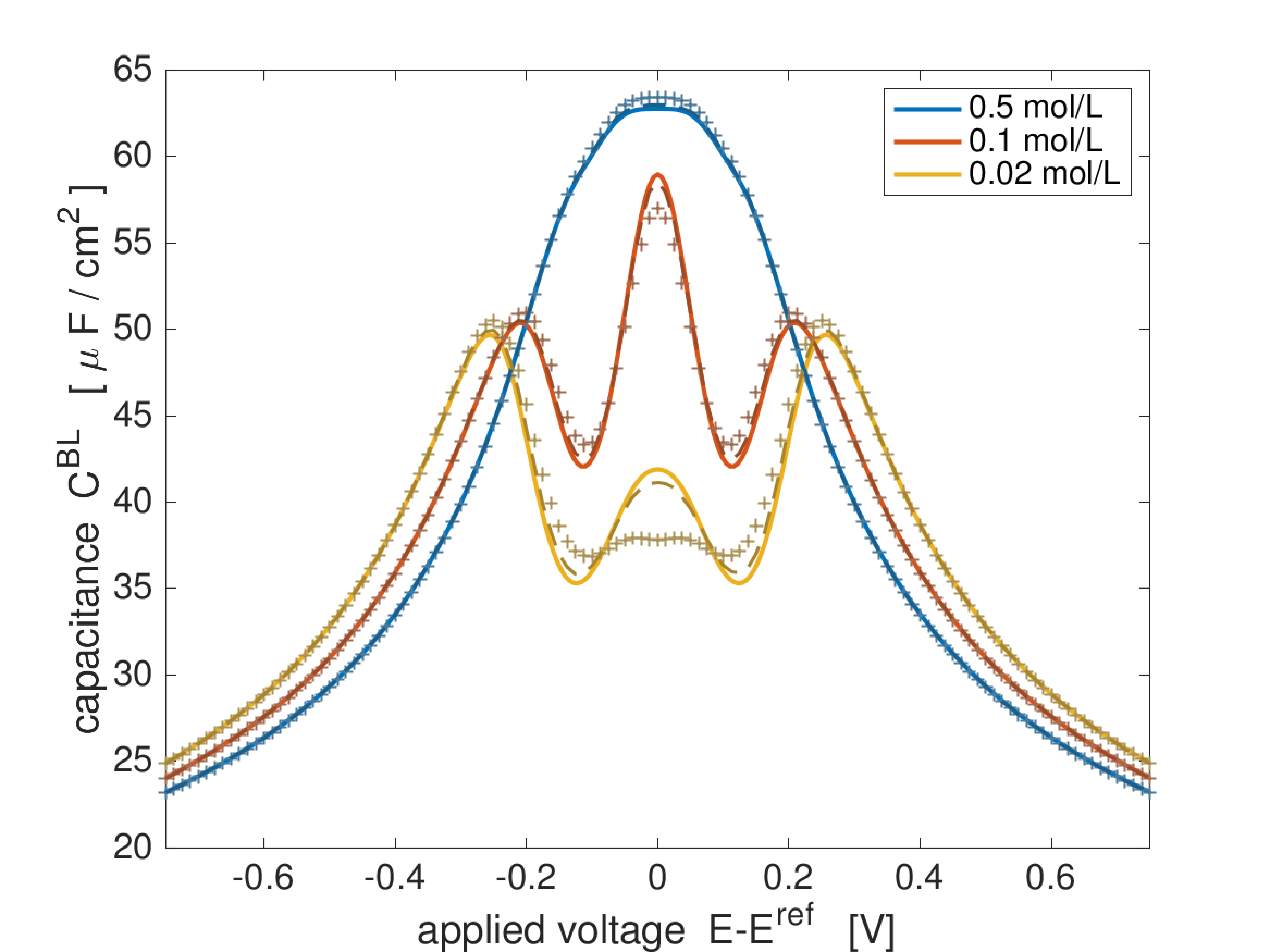
Left: profile of the electrostatic potential for a bi-crystalline interface with different grain sizes. Right: double layer capacity curves for different electrolyte concentrations. Solid lines (—) refer to the weighted sum of the grain contributions., (+) and dashed (- -) lines mark data obtained from numerical simulations for small and large grain sizes, respectively [1]
Non-constant Susceptibility in quasi-equilibrium system
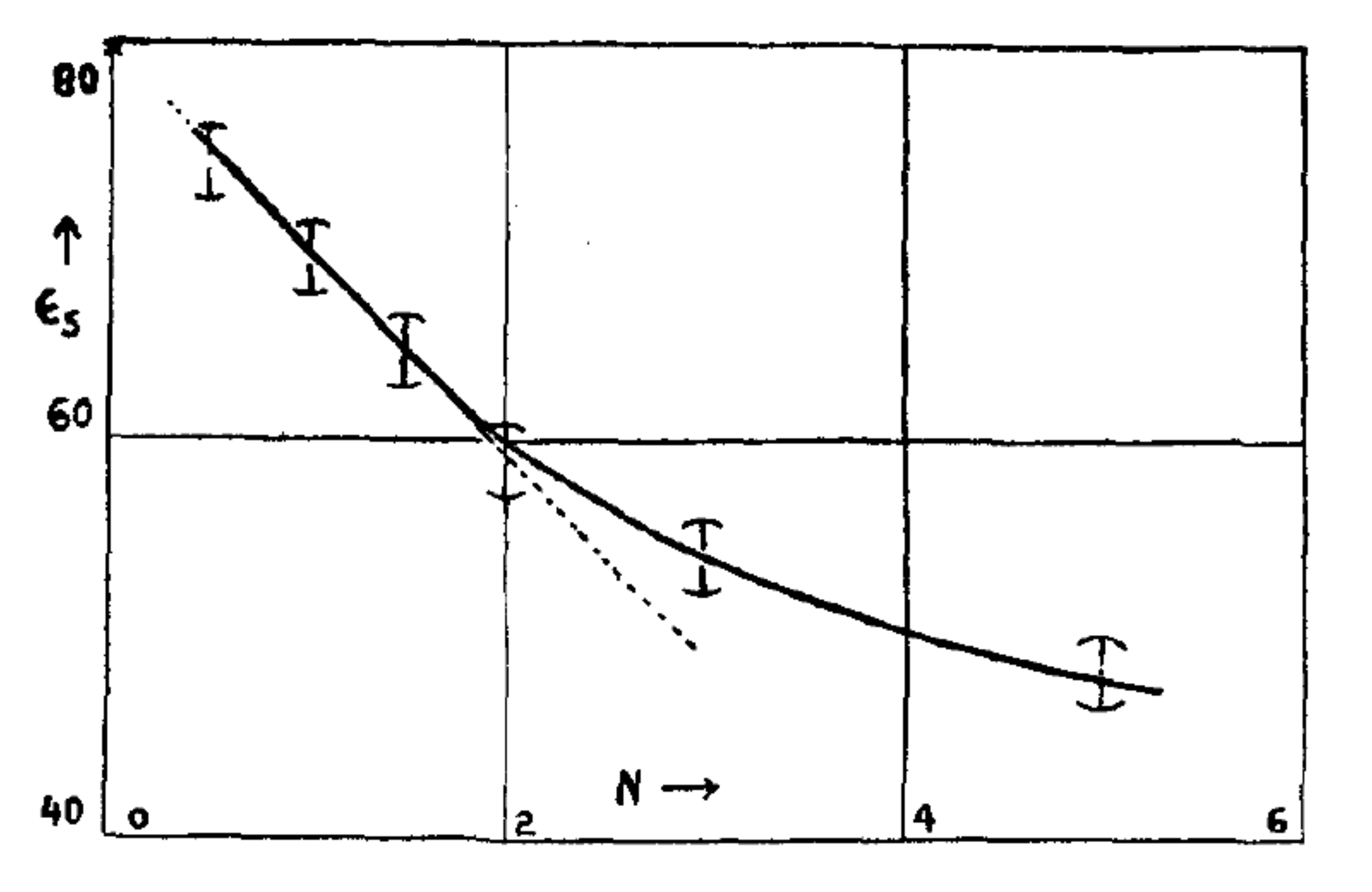
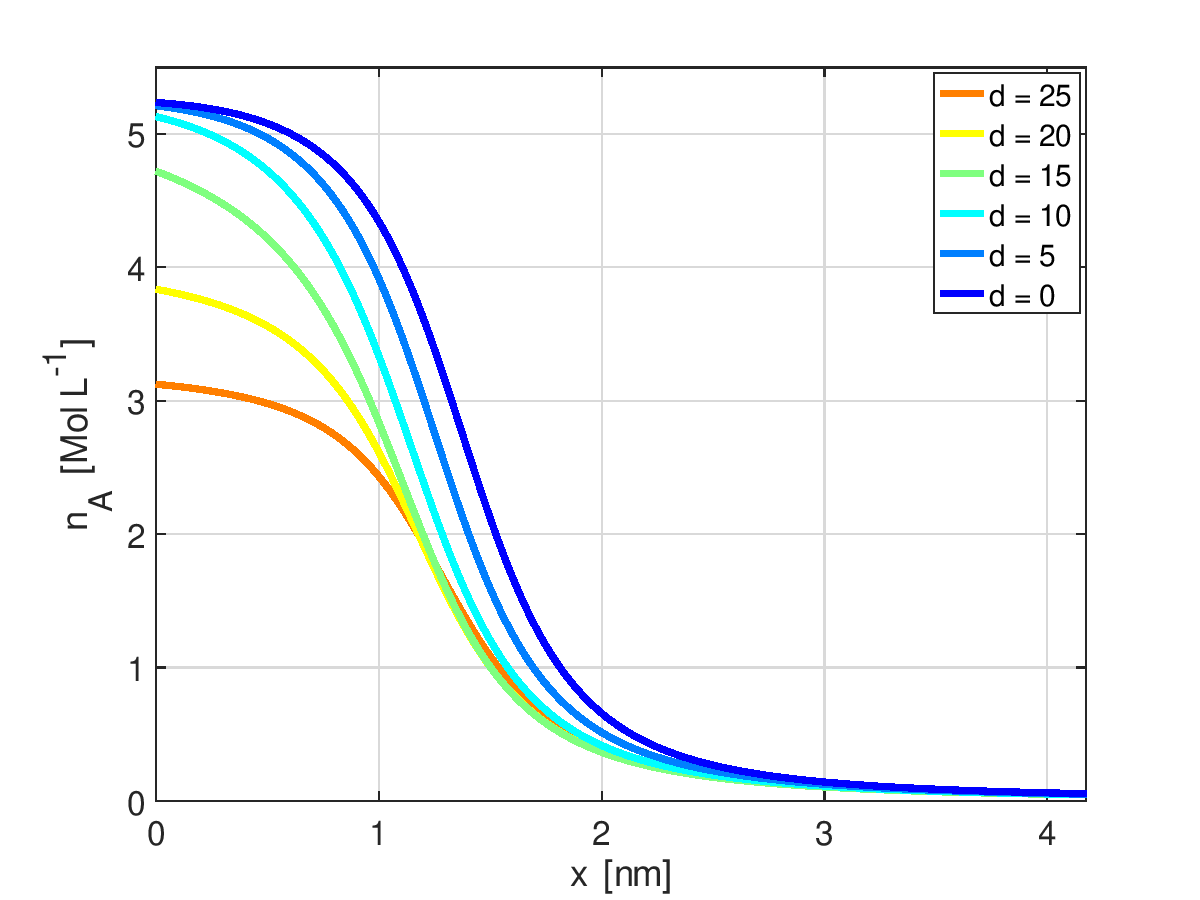
Concentration dependent susceptibility [2] (left) and spatial profiles of ion concentration in the boundary layer depending on the dielectric decrement [3] (right)
Concentration dependent susceptibility \(\chi = \sum_{\alpha\in \mathcal I} \chi_\alpha^{ref}\, v_\alpha^{ref} n_\alpha\) causes electric field dependent contributions to chemical potentials \(\mu_\alpha\) and pressure \(p\).
Consequences are: - non-obvious change in mathematical problem structure - reduction of ion accumulation in boundary layer - lower local capacitance maxima - fit to experimental data implies smaller solvation numbers of ions
Results for the nonequilibrium case
Finite volume schemes for Nernst-Planck-Poisson with ion volume constraints
See [4],[5]
- excess chemical potential and electrostatic potential gradients lumped into convective force Exact local and global charge conservation
- Consistency to thermodynamic equilibrium (“well
posedness”)
- On the continuous level, zero currents lead to (generalized) Poisson-Boltzmann equation.
- Proven discrete equivalent
- Decay of relative free energy along solution trajectories on approach to equilibrium for discrete counterpart of free energy
- Concentrations stay within (Nonnegativity, maximum concentration from volume constraint)
- Existence of solutions of discretized system
- Weak convergence of discrete solution to continuous solution
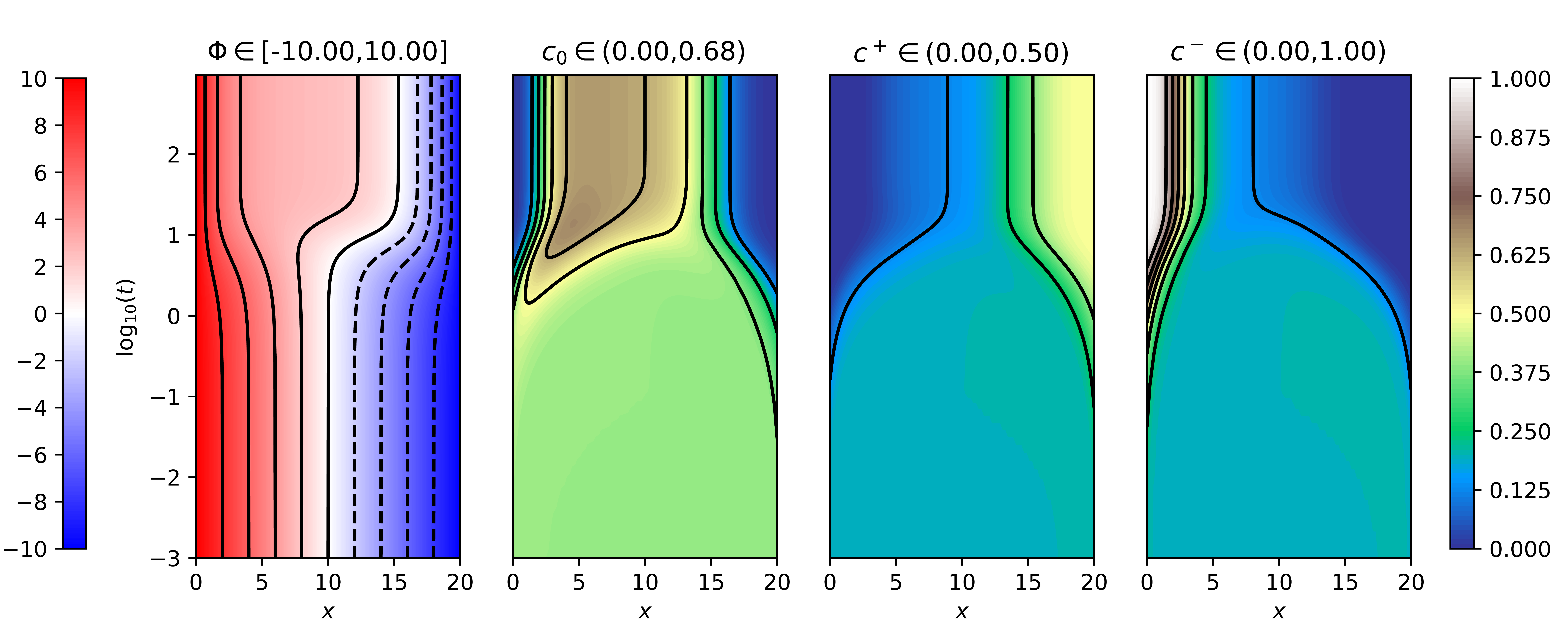
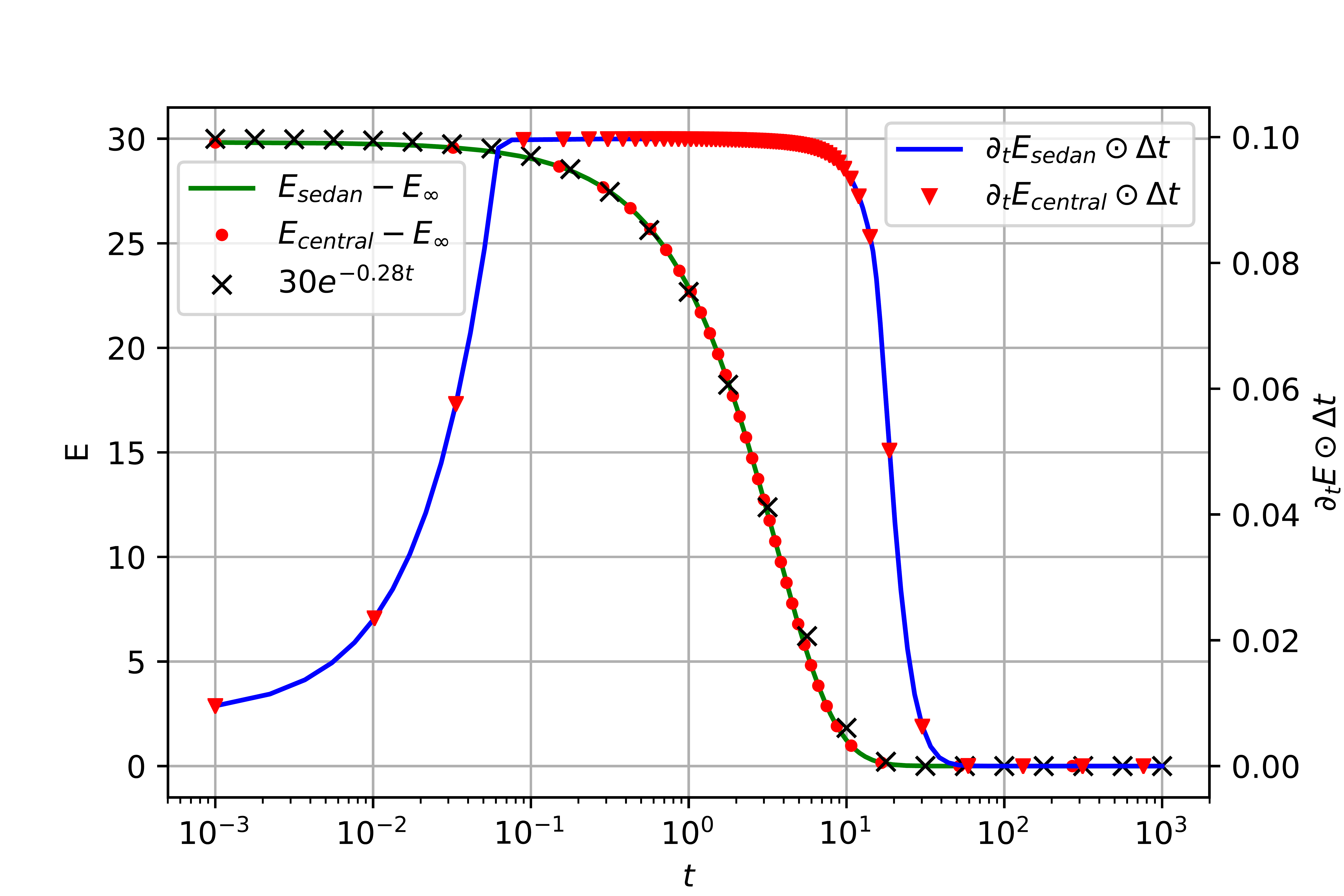
Left: Evolution of electrostatic potential and concentrations in a symmetric blocking cell with large cations. Right: Evolution of relative free energy and Energy dissipation per timestep [5].